In Situ Biogeochemical Transformation Processes
On this page:
- Schematic
- Introduction
- Other Technology Names
- Description
- Development Status
- Applicability
- Cost
- Duration
- Implementability Considerations
- Resources
Schematic
In Situ Biogeochemical Transformation Processes Schematic
Introduction
In situ biogeochemical transformation (ISBGT) refers to the abiotic transformation of contaminants by iron-containing minerals. Iron-containing minerals either occur naturally in the soil matrix or can be formed by microbial activity. ISBGT processes contribute to natural attenuation of chlorinated solvents at sites and can also be engineered in situ.
Other Technology Names
Abiotic Transformation
Biogeochemical Reductive Dechlorination (BiRD)
Biologically Mediated Abiotic Degradation (BMAD)
Description
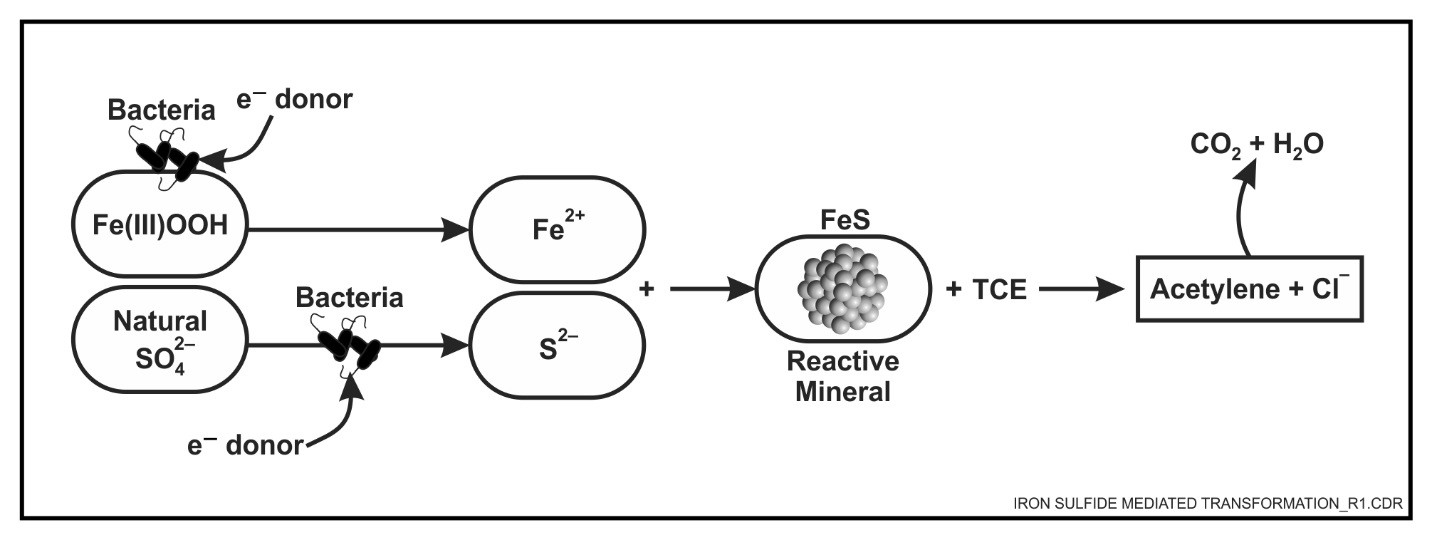
ISBGT processes result in the degradation of contaminants through combined biological, mineral, and chemical pathways. The contaminants are typically transformed abiotically by iron(II) minerals formed either by microbial activity or present naturally as part of the site geology (Environmental Security Technology Certification Program [ESTCP]), 2010). The minerals capable of abiotic contaminant transformations are iron sulfides (mackinawite [FeS], pyrite, greigite) and additional iron(II) minerals such as magnetite, green rust, and phyllosilicate clays (biotite and vermiculite) (EPA, 2009).
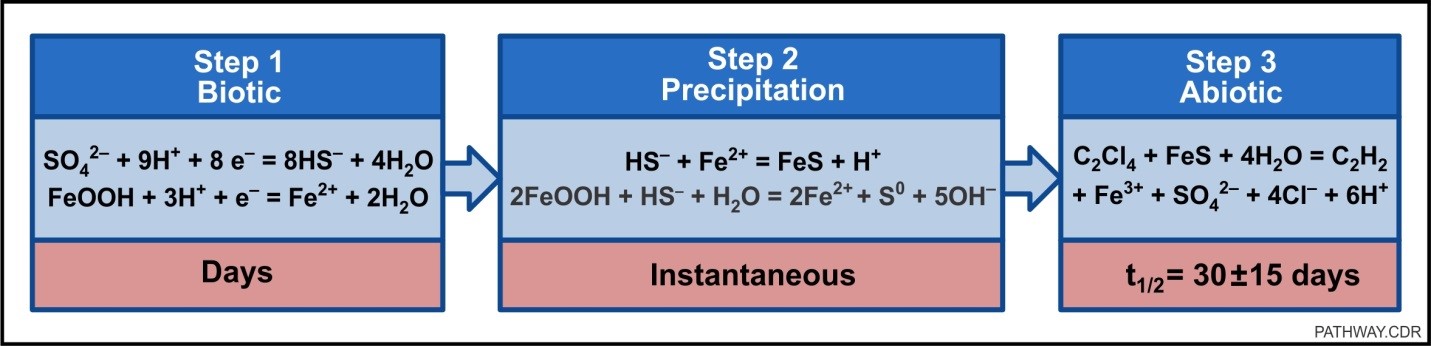
Formation of the iron sulfide species and the subsequent transformation of contaminants such as chlorinated solvents occur through multiple steps (NAVFAC, 2014). As shown in the figure below, the first biotic step consists of bisulfide (HS-) generation by sulfate-reducing microorganisms from naturally-occurring or amended sulfate, and parallel iron(II) generation from naturally-occurring iron(III) oxyhydroxides by iron-reducing microorganisms. The second step results in the rapid precipitation of amorphous and reactive iron sulfide species. In the third and final step, contaminants are abiotically transformed in the presence of the reactive iron sulfide species as electrons are donated from the iron sulfide to the chlorinated solvent. The primary mechanism is β-elimination (also known as dihaloelimination), where chlorines are lost from both carbons at the same time to form a carbon-carbon (C-C) bond leading to the formation of acetylene. The traditional sequential formation of lower chlorinated transformation products is a minor abiotic pathway. Formation of significant concentrations of lower chlorinated transformation products is a signature of the biotic, reductive dechlorination pathway.
Known characteristics of ISBGT are:
- It is a surface catalyzed reaction - once the surface has reacted, the reactivity of the mineral ceases (EPA, 2009).
- Reactivity is enhanced by the presence of iron(II) on the surface of the mineral (EPA, 2009).
- There is potential for iron(II) and iron(III) cycling on the surface of the mineral to sustain the transformation.
- Chlorinated solvents such as perchloroethylene (PCE) and trichloroethylene (TCE) are transformed directly to acetylene with minimal formation of cis-dichloroethylene (cis-DCE) and vinyl chloride (VC). This is favorable because of the tendency for cis-DCE stall and the carcinogenic nature of VC (NAVFAC, 2015).
- The transformation product acetylene is difficult to measure in the field (ESTCP, 2008).
- Degradation rates are often higher in slightly alkaline pH systems (EPA, 2009).
Abiotic Attenuation Processes Related to ISBGT
ISBGT can contribute to monitored natural attenuation of chlorinated solvents. However, as a process separate from ISBGT, iron minerals such as pyrite, magnetite, and biotite, naturally present in the soil matrix, can abiotically transform chlorinated solvents to acetylene when the chlorinated solvent comes into contact with the mineral surface. This degradation pathway was demonstrated in a microcosm study where soil from a site high in magnetite showed the same magnitude of transformation in both live and sterile microcosms (NAVFAC, 2015).
Engineering ISBGT
Engineering ISBGT has been limited to amending the treatment area to generate iron monosulfide as most research has been done on the reactivity of iron sulfides. To engineer ISBGT, three main components are necessary: sulfate, iron and electron donor. Sulfate- and iron-reducing microbes are typically ubiquitous and do not need to be amended into the treatment area. Engineering ISBGT can be accomplished in shallow plumes by amended permeable reactive barriers (PRBs) and in deeper plumes by the injection of liquid amendments (NAVFAC, 2015). The amendments are added to supplement the site conditions. For instance, sites that are high in sulfate but low in electron donor would be amended with electron donor. For PRBs, a trench is installed and typically solid amendments added, including mulch to provide an electron donor, gypsum to provide sulfate, and iron sand or hematite to provide iron. For liquid injection of amendments, dissolved organic substrate (e.g., sodium lactate) is used to provide electron donor and dissolved sulfate (e.g., magnesium sulfate) to provide sulfate; iron sulfate can be used to provide both sulfate and iron. Iron chloride has also been used to supply iron. If the alkalinity of the site is not sufficient, a buffer can also be added. Injections can be accomplished through permanently installed injection wells or by direct injection points (NAVFAC, 2014 and 2015).
Monitoring for ISBGT
When monitoring for the performance of amendments at enhancing ISBGT or when monitoring a site for the potential for ISBGT, a multidisciplinary approach must be taken. Analyses should include the groundwater and soil geochemistry. Detection for and analysis of the microbial community also should be done. The table below highlights the analyses that should be considered.
Groundwater Geochemistry |
Soil Analysis |
Microbial Analysis |
Dissolved Oxygen |
Bioavailable Iron |
Iron-Reducing Microorganisms |
Development Status and Availability
The following checklist provides a summary of the development and implementation status of ISBGT:
☐ At the laboratory/bench scale and shows promise
☐ In pilot studies
☒ At full scale
☐ To remediate an entire site (source and plume)
☐ To remediate a source only
☐ As part of a technology train
☐ As the final remedy at multiple sites
☐ To successfully attain cleanup goals in multiple sites
The amendments used to enhance ISBGT are available through the following vendors:
☒ Commercially available nationwide
☐ Commercially available through limited vendors because of licensing or specialized equipment
☐ Research organizations and academia
Applicability
|
Contaminant Class Applicability Rating for ISBGT (Rating codes: Demonstrated Effectiveness, ◐ Limited Effectiveness, No Demonstrated Effectiveness, ♢ Level of Effectiveness dependent upon specific contaminant and its application/design, I/D Insufficient Data) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
Nonhalogenated VOC |
Halogenated VOC |
Nonhalogenated SVOC |
Halogenated SVOC |
Fuels |
Inorganics |
Radionuclides |
Munitions |
Pesticides |
Emerging Contaminants |
| ○ | ● | ○ | ● | ○ | ● | ○ | ● | ◐ | ○ |
ISBGT has been shown to be capable of treating a wide range of inorganic and organic contaminants. Contaminant classes for which ISBGT has been successfully demonstrated include halogenated VOCs, halogenated SVOCs, inorganics and munitions. Each contaminant class is described in further detail below.
For halogenated VOCs and SVOCs, both engineered and natural systems with FeS have been shown to be capable of degrading chlorinated ethenes such as PCE, TCE and DCE with limited evidence of effectiveness with VC. The dominant degradation products from PCE and TCE in the presence of green rust was acetylene at 52% and 68%, respectively (SERDP, 2009). The general mechanism of degradation of chlorinated ethenes in the presence of the iron sulfides is dihaloelimination through chloroacetylenes to acetylene. The chlorinated ethanes 1,1,1-TCA, 1,1,2-TCA, 1,1-DCA, and 1,2-DCA, were degraded to lower chlorinated ethanes by reductive dichlorination or to chlorinated ethenes by dehydrohalogenation. Carbon tetrachloride is the only chlorinated methane that seems to be degraded by the iron sulfide minerals with chloroform being the dominant degradation product, where one chlorine is replaced by hydrogen (EPA, 2009).
Inorganic contaminants such as lead and arsenic can be precipitated and stabilized as metal sulfides or metal sulfide complexes during ISBGT (NAVFAC, 2014). At a federal site addition of an electron donor as mushroom compost to the soil promoted the conversion of sulfate to sulfide, then precipitation of the lead as lead sulfide. Leachability studies in the laboratory indicate that the leachability of metals stabilized as metal sulfides is greatly reduced.
Munitions constituents have been shown to be degraded in the presence of iron(II) coated magnetite, goethite and green rust in laboratory studies (EPA, 2009).
Limited laboratory studies exist in the literature showing the degradation of halogenated hydrocarbon pesticides such as ethylene dibromide (EDB) and hexachlorocyclohexane (HCH) (EPA, 2009). In the presence of FeS, EDB was abiotically degraded to ethylene. The reaction rates were lower than that of TCE with FeS. In just one study, HCH degraded to lower chlorinated cyclohexanes in the presence of FeS (EPA, 2009).
Sites where ISBGT can be found naturally occurring are typically sites where iron minerals such as pyrite and magnetite are found in the soil matrix. Engineering ISBGT (NAVFAC, 2014 and 2015) may be possible at sites where:
- Iron-containing minerals are present in the soil matrix.
- Naturally available dissolved sulfate is present in the groundwater.
- Naturally available dissolved Fe(II) is present in groundwater or solid Fe(III) in soil.
- Sufficient dissolved organic matter is necessary to maintain iron-/sulfate-reducing conditions.
- Iron- and sulfate-reducing microbes are present.
- Reaction rates are higher at slightly elevated pH.
Cost
ISBGT is an active remediation technology like enhanced in situ bioremediation (EISB). The major cost drivers are typically installation of wells and/or installing a trench for shallow contaminated sites. The amendments used are often reasonably priced but as with all in situ technologies, application costs vary according to site conditions and contaminants. Depending on the size of the contaminated area, labor and analytical costs may be substantial. Major cost items can be divided into two categories including upfront costs and operational and maintenance (O&M) costs. These cost categories along with some of the more common factors that impact individual cost components are provided below.
Upfront Costs
- Need for pilot studies or bench-scale tests to demonstrate effectiveness at a particular site
- Selection of suitable reactive material for barrier or amendment for liquid injections
- Well Installation for monitoring and also for injection of liquid amendments
- Trench installation for barrier configuration
- Injection manifold for injection of liquid amendments
Operation and Maintenance Costs
- Sample collection and analysesWetland size and number of units
- Additional injection/addition of amendments
Some of the best management practices for application of ISBGT are injecting the amendments in a ratio that allows sulfate to be in excess. This prevents the geochemistry of the treatment area to become methanogenic which promotes biological degradation. In terms of green and sustainable remediation (GSR) practices, the amendments are biodegradable; however, use of a solar-powered injection system is ideal to reduce energy use during the injection of amendments.
The list above highlights those cost dependencies specific to ISBGT and does not consider the dependencies that are general to most in situ remediation technologies. Click here for a general discussion on costing which includes definitions and repetitive costs for remediation technologies. A project-specific cost estimate can be obtained using an integrated cost-estimating application such as RACER® or consulting with a subject matter expert.
Duration
Since ISBGT does not rely on microbes to become mature before contaminant concentration decreases can be seen, often the effect of the amendments can be detected within the first 90 days after injection/placement of amendments. Monitoring after implementation of ISBGT will need to be conducted for 1 to 5 years before a reinjection or replacement of amendments is conducted. The expected cleanup time of ISBGT depends on several factors:
- Cleanup goals
- Plume size
- Contaminant concentrations and distribution
- In situ characteristics including permeability and anisotropy
- Amendment characteristics
- Groundwater geochemistry
Implementability Considerations
The following factors may limit the applicability and effectiveness of engineering ISBGT (ESTCP, 2015):
- Sites with low sulfate concentration and therefore no constant flux of sulfate
- Organic carbon content should be sufficient to promote sulfate-reducing conditions
- Sufficient iron is necessary to promote formation of iron sulfides
- Subsurface heterogeneity can interfere with uniform distribution of amendments
- Low-permeability soils are difficult to treat
- Sites with multiple contaminants, some of which are not treatable by ISBGT
Resources
EPA. Identification and Characterization Methods for Reactive Minerals Responsible for Natural Attenuation of Chlorinated Organic Compounds in Ground Water (2009)
This report describes the application of abiotic processes to remediate contaminants such as halogenated hydrocarbons. The specific minerals involved in the abiotic processes are described in detail.
ESTCP. Workshop on In Situ Biogeochemical Transformation of Chlorinated Solvents (2008)
This report is a summary of a workshop where researchers in the field of ISBGT met to discuss the state of the science related to ISBGT. During the workshop key questions were discussed and the collective responses to the key questions are provided in this document.
ESTCP. Enhanced Oxidative Bioremediation of cis-Dichloroethene (cis-DCE) and Vinyl Chloride (VC) using Electron Shuttles (2010)
This report is a summary of laboratory studies conducted to better understand the ISBGT of cis-DCE and VC.
ESTCP. In Situ Biogeochemical Treatment Demonstration: Lessons Learned from ESTCP Project ER-201124 (2015)
This report provides the results and lessons learned from a series of column studies and a field demonstration at Nike PR-58 site in North Kensington, Rhode Island conducted to better understand ISBGT. The report provides valuable information to help better understand how to implement ISBGT to treat contaminants.
NAVFAC. Biogeochemical Transformation Handbook (2015)
This handbook is a follow up to the NAVFAC 2014 fact sheet and provides a more in-depth introduction to ISBGT.
NAVFAC. In Situ Biogeochemical Transformation Processes for Treating Contaminated Groundwater (2014)
This factsheet provides an introduction to ISBGT and a general overview of the contaminants treated by ISBGT and how ISBGT can be implemented at a contaminated site.
SERDP. Abiotic Reductive Dechlorination of Tetrachloroethylene and Trichloroethylene in Anaerobic Environments (2009)
This report is a summary of one of the first studies where abiotic transformation of chlorinated solvents by minerals was observed in the laboratory.