Disinfection for Beneficial Reuse
On this page:
- Schematic
- Introduction
- Other Technology Names
- Description
- Development Status
- Applicability
- Cost
- Duration
- Implementability Considerations
- Resources
Schematic
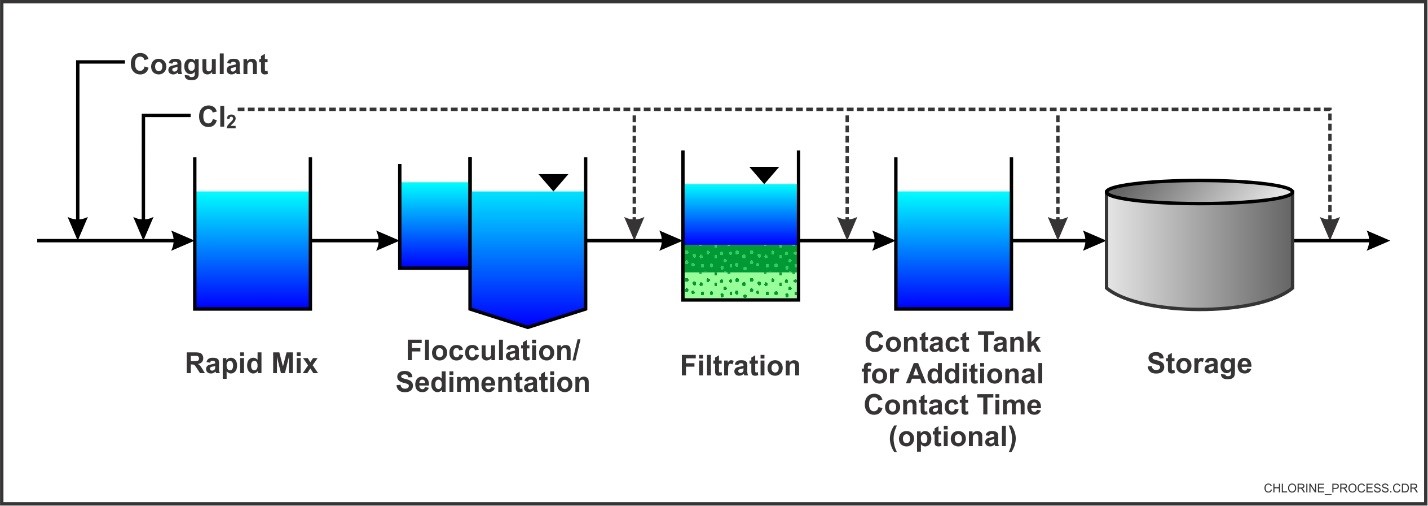
Typical Chemical Disinfection Application with Chlorine (Source: EPA)
(Note: Depicts General Wastewater Treatment Schematic; Groundwater Treatment Unit Processes Will Vary Based on Contaminant and Water Quality; Chlorine May Be Added for Oxidation Purposes and/or to Control Microbial Growth at Various Points in the Process per EPA, 2016b)
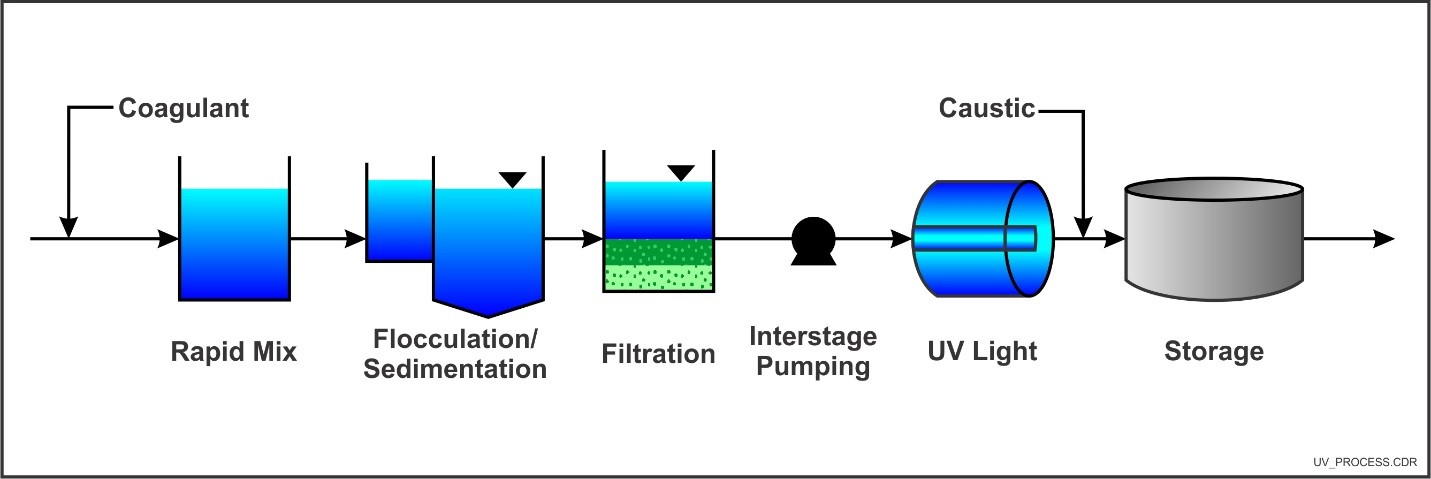
Typical Ultraviolet Disinfection Application (Source: EPA)
(Note: Depicts General Wastewater Treatment Schematic; Groundwater Treatment Unit Processes Will Vary Based on Contaminant)
Introduction
Treated groundwater or wastewater from remediation processes may require disinfection prior to beneficial reuse. This profile includes considerations for the disinfection of groundwater, as well as wastewater that may be generated from various groundwater treatment processes (such as backwash from filters or biological reactors).
Disinfection involves contacting a chemical agent and target microorganisms and viruses (referred to as pathogens) in the source water to eliminate the potential for the spread of waterborne disease. This is accomplished through the removal and/or deactivation of disease-causing pathogens. Chlorine is most commonly used for disinfection purposes, but other methods are available. Alternate methods that are commercially available include primarily chloramination, chlorine dioxide, ozone, ultraviolet disinfection systems, and combinations of oxidants. Disinfection is just one consideration for beneficial reuse as several water quality requirements will need to be met for the reduction of both microbiological and chemical contaminants prior to water reuse.
Other Technology Names
Chlorination
Chloramination
Chlorine
Chlorine Dioxide
Ozone
Ultraviolet (UV) Disinfection
Mixed or Combined Oxidant Processes
Description
Source water such as surface water or groundwater may contain pathogens that cause diseases in humans. This could include bacteria, viruses, protozoa (such as Cryptosporidium), or other parasites. If these pathogens are present, the source water must be adequately treated and disinfected prior to use as a potable water supply or for another beneficial reuse (e.g., irrigation). The disinfection process includes the removal and/or deactivation of pathogens in the source water as described below. Pathogens that are regulated for their elimination from drinking water due to human health concerns include fecal coliform and other fecal indicators, Cryptosporidium, Giardia Lamblia, Legionella, and Enteroviruses (EPA, 2016a). Disinfection is just one consideration for beneficial reuse as several water quality requirements will need to be met for the reduction of both microbiological and chemical contaminants. Water quality parameters to be tested for non-potable water reuse include biological oxygen demand, total suspended solids, nitrogen, phosphorus, turbidity, bacteria, and other site-specific constituents of concern. Water quality for potable reuse must meet all applicable drinking water standards.
Pathogens can be associated with high turbidity in water. Groundwater is typically low in turbidity, but wastewater from remediation processes can exhibit high turbidity and/or groundwater systems may be under the direct influence of surface water. Turbidity removal is an important first step in the elimination of pathogens. High turbidity levels can interfere with disinfection by increasing oxidant demand and/or encasing the pathogens in particles which shield them from oxidation or radiation. Turbidity can also promote microbial growth by providing a surface for attachment. The removal of pathogens may include the use of coagulation, sedimentation, and filtration or other filtration processes such as membrane filtration. Turbidity standards in treated water have been set for surface water (or groundwater systems under the direct influence of surface water) and should be consulted based on the type of filtration used at a given site (EPA, 2010). Reliable pathogen control for the beneficial reuse of treated water can be achieved by eliminating high turbidity and by providing for adequate disinfection. For more information on these turbidity removal processes, additional pages can be consulted such as the precipitation/coagulation/flocculation technology profile.
Once the turbidity has been removed and/or is already low in the groundwater source, various agents can be applied to inactivate the pathogens. These agents may include chlorine, chloramines, chlorine dioxide, ozone, or ultraviolet radiation. The inactivation process destroys the pathogen through oxidation or radiation and disrupts its ability to function, grow, and/or replicate. More information is provided below on the application of each of these disinfecting agents (EPA, 1999 and EPA, 2016b):
- Chlorine: Chlorine can be applied as free chlorine [Cl2], sodium hypochlorite [NaClO], or calcium hypochlorite [Ca(OCl)2]. It is an oxidant that can destroy pathogens, but also reacts with natural organic matter, ammonia, and other elements in the source water. The chlorine demand of the source water must be exceeded. This ensures that a residual level of chlorine can be maintained as needed based upon the water reuse application.
- Chloramination: Chlorine and hypochlorous acid react with ammonia to form chloramines. Chloramines include monochloramine (NH2Cl), dichloroamine (NHCl2) and trichloramine (NCl3). Chloramines may be used as an alternative to chlorine where high levels of natural organic matter exist in the source water to minimize the formation of halogenated disinfection byproducts (DBPs). The chloramine residual is more stable and persistent than free chlorine, which is one of the reasons that many water utilities have made the switch from using free chlorine to chloramine for disinfection of drinking water.
- Chlorine Dioxide: Chlorine dioxide (ClO2) is applied as a gas and is typically produced on site. It yields lower levels of organic DBPs than free chlorine, while maintaining a residual.
- Ozone: Ozone (O3) is applied as a gas and is typically produced on site. It is especially effective against Giardia and Cryptosporidium, although it does not produce a residual for downstream disinfection like chlorine.
- Ultraviolet (UV) Disinfection: UV radiation can also be used to achieve disinfection in a source water given sufficient light intensity and contact time. Cryptosporidium is susceptible to UV radiation, while chlorine is largely ineffective against this protozoan. UV disinfection does not provide any residual for downstream disinfection (EPA, 2006). One recent innovation is the use of UV systems that rely upon light-emitting diodes (LEDs) which are more energy efficient and provide potential costs savings over the operational life cycle (Song et al., 2016).
- Hydrogen Peroxide in combination with UV and/or Ozone: Hydrogen peroxide in combination with UV and/or ozone generates hydroxyl radicals, which are more effective than UV or ozone alone.
Development Status and Availability
The following checklist provides a summary of the development and implementation status of disinfection technologies:
☐ At the laboratory/bench scale and shows promise
☐ In pilot studies
☐ At full scale
☐ To remediate an entire site (source and plume)
☐ To remediate a source only
☒ As part of a technology train
☐ As the final remedy at multiple sites
☐ To successfully attain cleanup goals in multiple sites
Disinfection technologies are available through the following vendors:
☒ Commercially available nationwide
☒ Commercially available through limited vendors because of licensing or specialized equipment
☐ Research organizations and academia
Applicability
This technology is applicable to source water containing pathogens that require treatment prior to water reuse applications. It may be required for groundwater extraction and treatment (GWET) systems where the end use of the water is for beneficial reuse.
The selection of a given disinfection system is dependent upon many factors including: the pathogens in the source water; the source water quality; the potential for DBP formation; ease of use and maintenance; health and safety considerations; building size; treatment plant capacity; pre-treatment requirements; and the overall cost.
Cost
Disinfection requires equipment procurement, installation and ongoing operation and maintenance costs primarily related to chemical or electrical costs. Major cost drivers include:
Upfront Costs
- Treatability tests - Bench-scale testing may be required to determine chemical dosages for disinfection (site-specific depending on the source water quality). Testing can also assess the formation potential for halogenated DBPs.
- Disinfection equipment must be purchased and installed including components below:
- Liquid Injection - pump, tank, piping, and injection point
- Gas Injection - pressurized gas cylinder, piping, and injection point
- On-Site Generation Equipment
- UV Lamps
- In-line mixers following liquid, or gas injection
- Secondary containment and associated requirements for storage, conveyance, and / or ventilation of hazardous chemicals used for disinfection and any necessary monitoring to ensure safe storage and use of these materials.
- Turbidity removal equipment (optional) - If turbidity removal is required, there will be capital costs for mixing, coagulant dosing, precipitation, and filtration.
- Labor will be required for on-site installation and will depend upon the location and capacity of the plant, facility requirements, and required personal protective equipment (PPE).
- Utilities must be installed to meet the equipment power supply needs.
Operation and Maintenance Costs
- Disinfection chemical supply - Chemicals will be required to dose disinfectants at target concentrations.
- Coagulant chemical supply (optional) - If turbidity removal is required, chemicals will be required to achieve coagulation/removal.
- Utilities - Power supply for pumps, on-site generators, or UV system.
- Labor (for operation and maintenance) - Location and capacity of plant; facility requirements; PPE.
- Equipment maintenance (e.g., pumps, UV lamps) - Regular maintenance of equipment per manufacturer specifications.
The list above highlights those cost dependencies specific to disinfection technologies. Click here for a general discussion on costing which includes definitions and repetitive costs for remediation technologies. A project-specific cost estimate can be obtained using an integrated cost-estimating application such as RACER® or consulting with a subject matter expert.
Duration
Disinfection is an ongoing and continuous process. It is required as long as the source water is being supplied for beneficial reuse.
Implementability Considerations
- Chlorine can promote the formation of halogenated DBPs, which have adverse health effects at high levels in drinking water.
- Other byproducts such as N-nitrosodimethylamine with chloramination, chlorite/chlorate with chlorine dioxide, and bromate with ozone may be formed in the treated water and can have adverse health effects at high levels in drinking water.
- Residual levels of disinfection do not form in ozone and ultraviolet systems.
- Ozone or ultraviolet treatment can be used if pathogens are resistant to chlorine (such as Cryptosporidium).
- Proper PPE and health and safety measures must be followed for storage and handling of chemical oxidants (EPA, 2016b).
Resources
AEPA. Alternative Disinfectants and Oxidants Guidance Manual (1999)
Provides technical data and engineering information on disinfectants other than chlorine.
EPA. Ultraviolet Disinfection Guidance Manual (2006)
Provides technology background information on UV light, microbial response to UV light, and UV reactors.
EPA. Comprehensive Surface Water Treatment Rules Quick Reference Guide: Systems Using Conventional or Direct Filtration (2010)
Provides guidance on turbidity removal via filtration processes.
EPA. National Primary Drinking Water Regulations: Microorganisms (2016a)
A table of microorganisms, treatment goals, potential health effects, and sources of contamination.
EPA. Drinking Water Treatability Database (2016b)
Presents technology information on the control of contaminants in drinking water.
Song, K., Mohseni, M., and F. Taghipour. Application of Ultraviolet Light-Emitting Diodes (UV-LEDs) for Water Disinfection: A Review. Water Resources 94: 341-349. (2016)
Reviews the use of UV-LED compared to traditional UV mercury lamps.
