In Situ Chemical Reduction
On this page:
- Schematic
- Introduction
- Other Technology Names
- Description
- Development Status
- Applicability
- Cost
- Duration
- Implementability Considerations
- Resources
Schematic
This information may be reproduced without restriction as long as the source attribution is included.
In Situ Chemical Reduction
Introduction
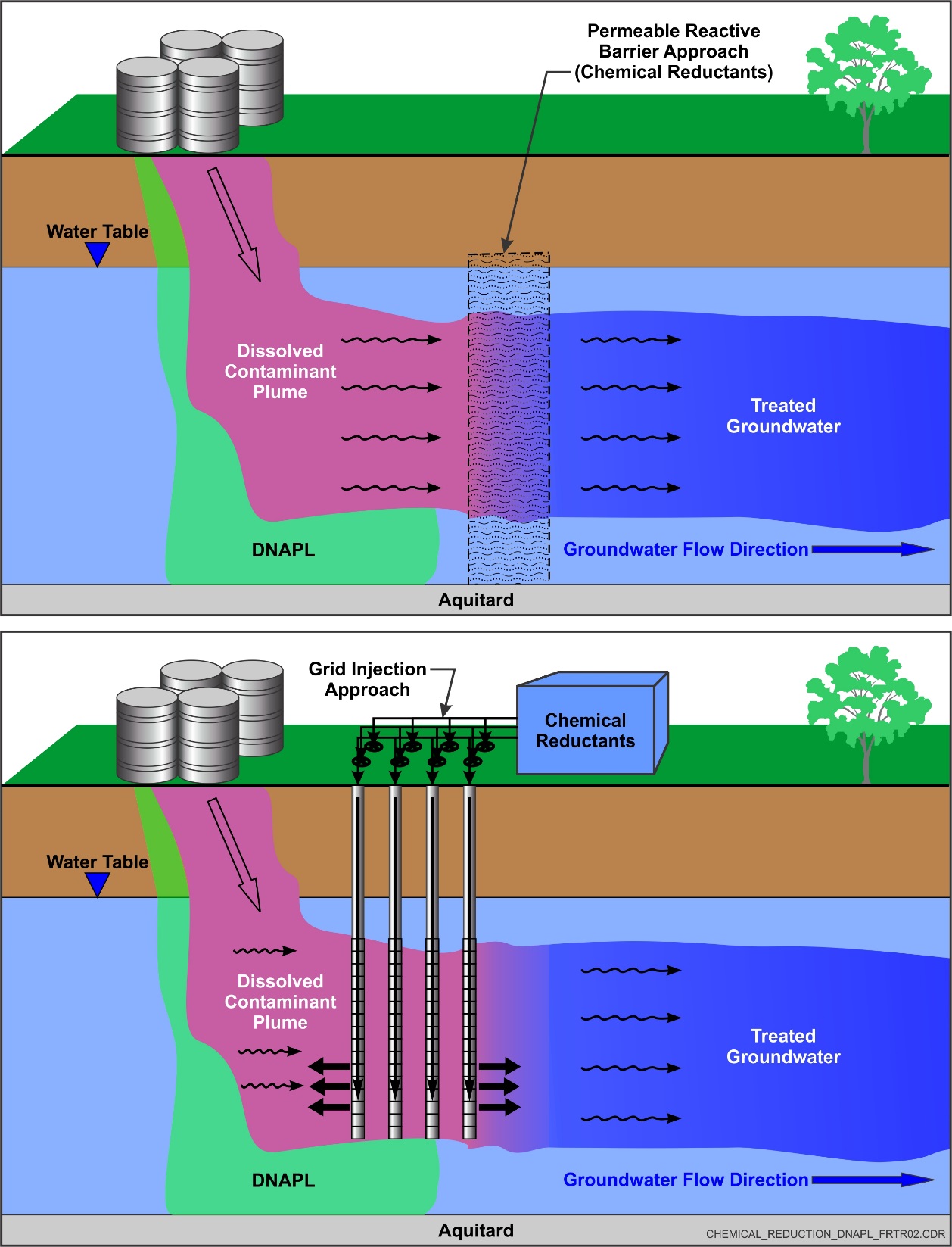
In situ chemical reduction (ISCR) is the in-place abiotic transformation of contaminants by chemical reductants. Contaminants treated by ISCR typically include chlorinated compounds (e.g., chlorinated solvents such as trichloroethene [TCE]), metals in a high oxidation state (e.g., hexavalent chromium or Cr6+), explosives (e.g., TNT, RDX, and HMX), and oxidized inorganics (e.g., perchlorate). Commonly used chemical reductants include zero valent iron (ZVI), zero valent zinc (ZVZ), iron minerals, bi-metallic materials, polysulfides, dithionite, and other proprietary commercial materials such as furnace slag or iron scrap. Hybrid amendments that combine ZVI emulsified in a carbon substrate are also frequently used to treat chlorinated compounds, which create strong reducing conditions to drive chemical reduction while also supporting reductive dechlorination. ISCR is used for soil and/or groundwater remediation, and can treat dissolved contaminants as well as halogenated dense non-aqueous phase liquids (DNAPLs). It is typically implemented through the construction of a permeable reactive barrier (to intercept contaminant plumes or by direct injection, soil fracturing and injection, or mixing into the subsurface soil to target source or highly contaminated zones.
Other Technology Names
Chemical Reduction
Description
ISCR refers to the category of in situ remediation technologies by which treatment occurs by chemical reduction of contaminants of concern (COCs) (Tratnyek et al., 2014).). Biogenic minerals, such as iron sulfides formed by the activity of microorganisms, also serve to reduce COCs, such as chlorinated ethenes. These biogeochemical transformation processes are described here. The reducing conditions necessary for ISCR are often created by adding chemical reductants, but also can arise from natural intrinsic biogeochemical processes or stimulating in situ microbial activity.
Common chemical reductants that contribute to ISCR include reduced metal species (e.g., ZVI, ferrous iron [Fe2+], and iron oxide [Fe3O4]), reduced sulfur species (e.g., bisulfide ion [HS-], iron sulfide [FeS] and dithionite [S2O42-]). These reductants are naturally occurring in minerals and natural organic matter (NOM), can be generated from natural biogeochemical processes, or are available as engineered reductants (e.g., ZVI and S2O42-). The engineered reductants can be introduced in the formation as dissolved solutions (sodium dithionite), non-aqueous phase liquids (emulsified vegetable oil), emulsions, foams (calcium polysulfide), gases (H2), and solids (micro- and nano-scale ZVI).
The reduction processes responsible for contaminant treatment by ISCR include degradation and sequestration. Contaminants that are subject to ISCR conditions include: 1) organic compounds with chloro-, nitro-, or other readily reducible functional groups (e.g., hydroxyl [-OH]); 2) high valent metal cations that become less mobile upon reduction such as Cr6+, which is reduced to Cr3+; and 3) oxidized nonmetal inorganics such as nitrate and perchlorate.
While ISCR has been applied to various contaminants using different reductants, much of the research, technology development, and applications have focused on the treatment of chlorinated and halogenated compounds using reduced metal species, and have identified the following characteristics (Brown, 2011):
- The reductive processes involve a form of reduced metal, typically ZVI or Fe2+.
- Abiotic, iron-mediated reductive pathways are generally different and more diverse than those that typically occur with biologically mediated reduction.
- Iron-based reductive processes are strongly surface and pH dependent.
- Iron-mediated reductive processes can be enhanced through the use of chemical and/or biological reduction.
Multiple reductive processes are involved in the ISCR of chlorinated solvents and the actual ISCR processes that take place in the treatment zone depend on the species of contaminant and the type of reducing agent being used. The natural biological processes that take place in the subsurface also affect the types of reductive processes. As a result, the carbon-based end products for ISCR treatment of chlorinated ethenes can vary from completely dechlorinated compounds (e.g., ethene from TCE) to carbon dioxide. In addition, complete degradation can occur through the beta (β)-elimination pathway to produce acetylene as described here. Halogen ions are typically one of the end products of this process.
Several variations of ISCR technologies have been established and are widely applied or are recently emerging and subject to further evaluation (SERDP-ESTCP, 2014). These include:
- Combined ISCR and in situ bioremediation using hybrid amendments have been developed that combine chemical reductants such as ZVI and materials that stimulate microbial activity (various forms of organic carbon). The amendments are available as commercial products that are applied through injection in the form of emulsified liquids or colloids through injection. Additional information pertaining to the design, costs, and implementability considerations for in situ soil mixing can be found here; amendments can be applied to both source zones and groundwater plumes to provide chemical reduction and promote biodegradation. Although the relative significance of abiotic versus biotic contaminant reduction by these amendments is difficult to quantify, applications of these amendments have been demonstrated to effectively reduce COCs at multiple sites.
- In situ biogeochemical transformation (ISBGT), or biogeochemical reductive dechlorination, is the process of stimulating abiotic reduction of chlorinated solvents by formation and reaction with iron sulfides. Iron sulfides are created by stimulating microbial sulfate reduction in the presence of iron. A source of carbon and sulfate generally must be added, and iron may be added, although naturally occurring iron minerals are often sufficient. Sulfate- and iron-reducing microbes are typically ubiquitous and do not need to be introduced into the treatment area. Additional information pertaining to the design, costs, and implementability considerations for in situ soil mixing can be found here.
- Catalytic reductive dechlorination (CRD) is dechlorination through hydrogenolysis catalyzed by noble metals (e.g., palladium [Pd]). Although CRD performs well in bench- and pilot-scale tests, deactivation of the Pd catalyst can occur, especially when the groundwater contains sulfide. However, deactivation is reversible upon treatment with a suitable oxidant such as sodium hypochlorite or oxygen-saturated water. Recent catalyst formulations are available that improve resistance to deactivation and increase the time needed before regeneration. One example is to incorporate gold (Au) clusters on Pd or use zeolite supports to separate the Pd from constituents in the water that deactivate the catalyst. Although there is a high capital cost of using Au and Pd and the applicability of this form of ISCR is limited, implementation of CRD using in-well recirculating reactors may be promising. Another example is the use of an inoculum mixture containing vitamin B12, cobalt, titanium citrate and a carbon source, which has been implemented successfully at full scale to treat groundwater contaminated with tetrachloroethylene (PCE), TCE, and tetrachloroethene.
- In situ redox manipulation (ISRM) consists of the application of moderately strong chemical reductants such as sodium dithionite or calcium polysulfide to reduce naturally occurring Fe3+ in the mineral matrix.1 The resulting Fe2+-bearing minerals serve as the reductant that reacts with metals, such as Cr6+. Sodium dithionate has shown potential to reactivate spent ZVI from previous injection events (Xie and Cwierny, 2010). However, to date, there are limited field data that demonstrate the efficacy of this application of this treatment.
ISCR can be used to treat source areas and to treat or contain the dissolved phase plume as follows:
Source Zone Treatment
ZVI emulsified within a carbon substrate has been designed to treat DNAPL source areas with some successful full-scale applications. These hybrid amendments have been designed to treat chlorinated DNAPL source areas, with the DNAPL becoming dissolved into the carbon substrate where it can be contacted by the emulsified ZVI for chemical reduction. However, the injection of chemical reductants to directly target source zone contamination, especially where residual or free phase DNAPL is present, has not been widely utilized to date. Hydraulic or pneumatic fracturing can be used to facilitate emplacement.
Although other forms of chemical reductants are feasible, micro-scale ZVI has been the most frequently used chemical reduction applications to date for targeted injections in the source zone. Nano-scale ZVI also has been used, but less frequently, due to its high cost and reactivity, which reduces its longevity. This approach tends to be more effective in relatively permeable lithologies with low fines content, which facilitates a homogenous distribution of amendment. In general, amendments are delivered to the subsurface using a grid approach as particulates, such as colloidal ZVI, or liquids and foams. Particulate slurry forms of reductant amendments are attractive as they may remain present and reactive in the source area for longer times than liquids and foams and therefore provide residual treatment capacity. For these reasons, source zone targeted injections (SZTI) using nano-scale ZVI has attracted more attention, but the fine particulate nature of this reductant raises other challenges related to longevity and emplacement. Injections of a carbon source or a reducing agent such as sodium dithionate have been routinely used to immobilize metals such as Cr6+ to Cr3+. Calcium polysulfide has also been used for the treatment of Cr6+ source zones. In this treatment method, calcium polysulfide foam is injected throughout the source zone to reduce Cr6+ to the less mobile Cr3+ form.
Source areas also can be treated by mixing soil with a variety of chemical reductants using large-diameter augers or retrofitted backhoes. The most prominent examples of applying ISCR using in situ soil mixing involves adding ZVI and clay. Typically, clay (1 to 2%) and micro-scale ZVI (0.5 to 2% on a weight basis of contaminated soil) are mixed into the contaminated zone soil matrix using large-diameter (4 to 8 feet) augers or soil mixers. Clay has the potential to disperse DNAPL as an emulsion and can also inhibit the movement of contaminated groundwater by decreasing overall aquifer permeability. Mixing improves contact between the emulsion and the ZVI. The main application of this technology has been DNAPL source zones. Additional information pertaining to the design, costs, and implementability considerations for in situ soil mixing can be found here.
Downgradient Plume Treatment and Containment
ISCR amendments are commonly used to treat dissolved phase plumes. Direct injection in a grid pattern can be used to treat large portions of the dissolved phase plume, whereas permeable reactive barriers (PRBs) may be installed to limit plume migration. Direct injection through wells or points is performed using micro- or nano-scale ZVI, whereas PRBs are generally constructed by creating a trench and applying granular ZVI, although direct injection of micro- or nano-scale ZVI also can be performed. Methods such as soil mixing and hydraulic or pneumatic fracturing also can be used to facilitate placement to treat portions of the dissolved phase plume or to install PRBs. Additional information pertaining to the design and implementation of PRBs can be found here and information pertaining to the introduction and distribution of amendments can be found here.
The above ISCR technologies, in terms of reductant strength and zone of treatment, are shown in the figure below to provide guidance for selecting an appropriate technology method. In general, technologies involving stronger chemical reductants capable of treating high contaminant concentrations are used to treat areas at or near the source, while technologies involving weaker chemical reductants are used to treat groundwater plumes downgradient of the source.
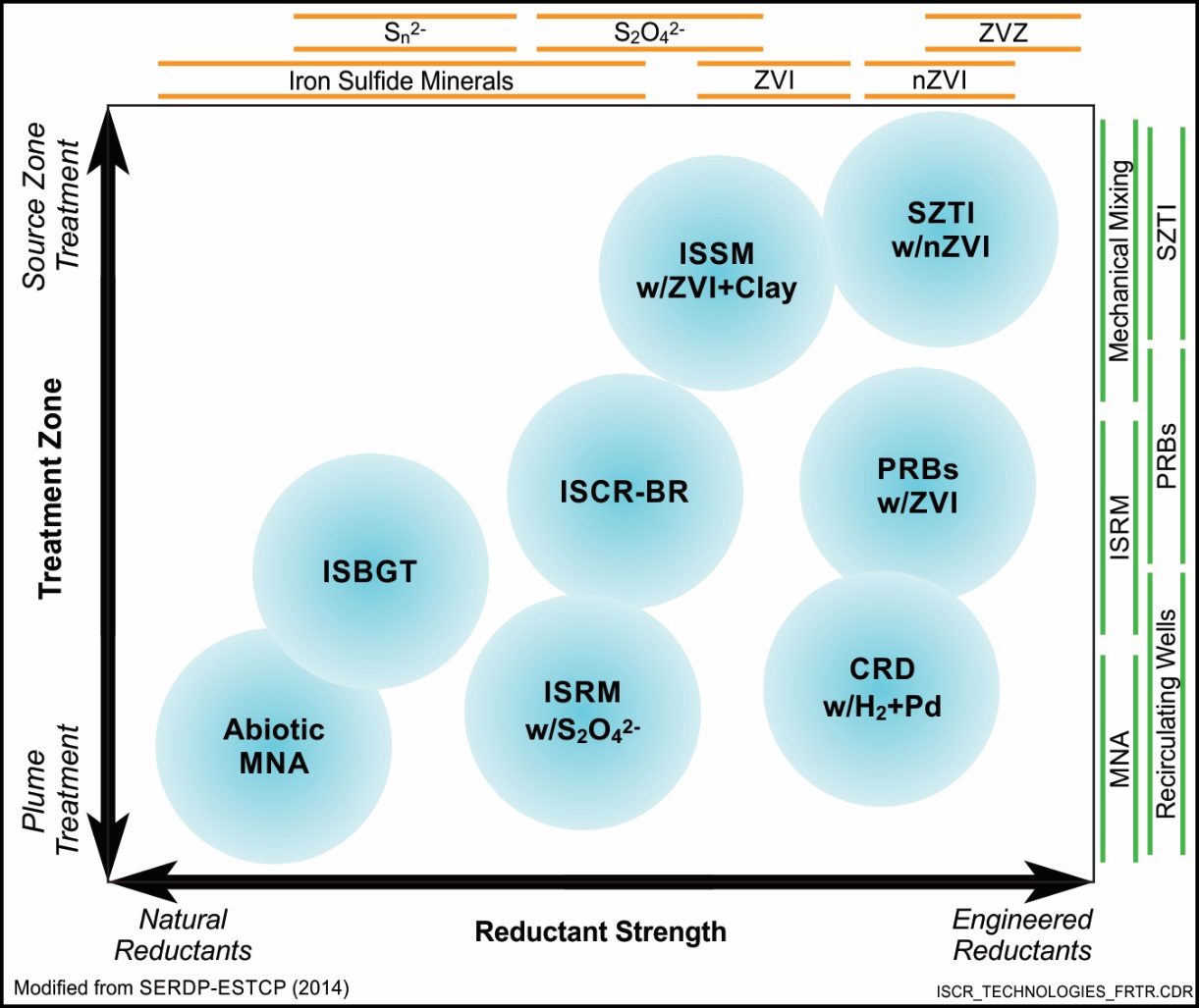
Applicability Considerations for ISCR Technologies
Development Status and Availability
The following checklist provides a summary of the development and implementation status of ISCR:
☐ At the laboratory/bench scale and shows promise
☒ In pilot studies
☒ At full scale
☒ To remediate an entire site (source and plume)
☒ To remediate a source only
☒ As part of a technology train
☒ As the final remedy at multiple sites
☐ To successfully attain cleanup goals in multiple sites
ISCR is available through the following vendors:
☒ Commercially available nationwide
☐ Commercially available through limited vendors because of licensing or specialized equipment
☐ Research organizations and academia
Applicability
|
Contaminant Class Applicability Rating for ISCR (Rating codes: Demonstrated Effectiveness, ◐ Limited Effectiveness, No Demonstrated Effectiveness I/D Insufficient Data, N/A Not Applicable) |
||||||||
|---|---|---|---|---|---|---|---|---|
Nonhalogenated VOC |
Halogenated VOC |
Nonhalogenated SVOC |
Halogenated SVOC |
Fuels |
Inorganics |
Radionuclides |
Munitions |
Emerging Contaminants |
| N/A | ● | N/A | ◐ | N/A | ● | N/A | ◐ | I/D |
ISCR technologies have been shown to be capable of treating halogenated volatile organic compounds (VOCs) and, to a lesser extent, halogenated semi-volatile organic compounds (SVOCs), metals (e.g., reduce Cr6+ to Cr3+), inorganics (e.g., perchlorate), and some munition constituents (e.g., RDX). The efficacy of technologies using engineered reductants, such as ZVI, are dependent on site hydrogeological conditions, while technologies based on natural or induced reductive reactions are also dependent on favorable site geochemical conditions or the addition of amendments.
Because of the wide variety of installation and delivery methods, ISCR technologies are applicable at a wide range of sites. Applications to treat DNAPL zones through soil mixing with large-diameter augers can reach soils at depths as great as 50 ft below ground surface. ISCR technologies can be applied in unconsolidated media to greater depths by injection through wells or open boreholes as well as in fractured rock systems. Hot spots can be treated with individual injections, and a plume can be cut off with a PRB-like treatment wall/trench or by injection through a line of wells or boreholes.
Cost
Cost drivers for ISCR technologies include the type and quantity of materials (chemical reductants or amendments, and infillings) required, and the delivery or emplacement methods needed, which are similar to other in situ technologies that rely on the application and distribution of amendments and are described here. Costs associated with emplacing ISCR amendments in a PRB can be found here. As with all in situ technologies, application costs vary according to site conditions and contaminants. Major cost drivers include:
Upfront Costs
- Areal extent and depth of contaminants. Extent of contamination impacts the number and depth of injection points, and therefore the quantity of chemicals or amendments required and the delivery or emplacement methods that can be selected.
- Nature of the contaminants and degradation pathways. The types of contaminants and their concentrations determine the type and quantity of amendments required and impacts the longevity of the materials.
- Geochemical conditions. For example, the presence of high nitrate, sulfate, and alkalinity levels can reduce the longevity of ISCR amendments, requiring more frequent injections of materials or replacement of PRBs.
- High resolution hydrogeologic studies to verify capture of the plume and prevent contamination bypassing the treatment zone.
- Chemical reductant remedial goals. More stringent remedial goals may require a greater amount of amendment volume or injection events.
Operation and Maintenance Costs
- Longevity of materials. Short-lived materials may require frequent replacement/replenishment. Longevity is impacted by material properties, types of contaminants, contaminant concentrations, type and content of soil minerals, groundwater geochemistry, and groundwater flow rate.
- Performance criteria. Performance criteria can impact the frequency that the aquifer must be replenished with additional materials to ensure proper treatment. Continuous performance monitoring is necessary to respond timely to any contingency detected.
- Changes in hydraulic conductivity. Periodic testing of hydraulic conductivity and/or water level monitoring of wells upgradient, within and downgradient of a PRB should be performed to assess whether the introduced materials are adversely impacting groundwater flow.
The list above highlights those cost dependencies specific to ISCR and does not consider the dependencies that are general to most in situ remediation technologies. Click here for a general discussion on costing which includes definitions and repetitive costs for remediation technologies. A project-specific cost estimate can be obtained using an integrated cost-estimating application such as RACER® or consulting with a subject matter expert.
Duration
Treatment times are similar to those required to implement in situ biological treatment such as reductive dechlorination, ranging from a few years to more than 10 years depending on remedial objectives and the implementation approach taken. For instance, source treatment using a closely spaced grid of injection points or wells will be shorter than a passive PRB designed to contain and prevent further downgradient migration of the dissolved phase plume, although the time required to achieve site cleanup goals following source treatment may still be significant unless additional remediation measures are implemented. The longevity of ISCR applications is dependent on many factors, including the following:
- Contaminant mass and distribution in the source area (affects source area ISCR applications)
- Groundwater flow rate and mass flux of contaminants (affects applications to treat the dissolved-phase plume)
- Soil heterogeneity, texture, and organic fraction content
- Aquifer geochemistry, particularly high nitrate, sulfate, and alkalinity levels
- Iron and sulfate availability and reaction rates
- Initial quantity and reactivity of the materials used in the ISCR applications and uniformity of distribution
- Type and size of reactive media used for ISCR application
- Media enhancement through sulfidation process
- Natural reductant demand impacting the materials use rate
In general, longevity of reductants such as ZVI is inversely related to its reactivity. Coarse-grained reductants exhibit less reactivity and persist longer than fine-grained materials due to the difference in surface area to volume ratio. The reactivity of iron minerals and coarse-grained ZVI can persist for several years in the subsurface. Conversely, nano-scale ZVI has a much greater surface area to volume ratio and reacts relatively fast in comparison to granular or micro-scale ZVI. The actual longevity of the ISCR materials under field conditions is not well known, and the ISCR materials and applications may need to be replenished or replaced as the chemical reductants are oxidized or passivated, the results of previous applications of ZVI slurries and emulsified ZVI suggest their effective lifetimes can be several years.
Implementability Considerations
Below are key considerations associated with implementing ISCR. Considerations common to technologies that rely on the introduction and distribution of amendments can be found here. Considerations pertaining to design and implementation of PRBs can be found here.
- The lateral and vertical extent of the source zone and contaminant plume influences the type of ISCR chosen and the method by which the amendments are introduced into the aquifer.
- For PRB- and injection-based technologies, the effective delivery of chemical reductants or amendments to the zone of contamination is crucial to the success of ISCR applications. For this reason, highly impermeable materials such as clay and bedrock matrix will limit the success of ISCR or require the use of permeability enhancement techniques such as hydraulic or pneumatic fracturing.
- ISCR applications involving the injection of ZVI slurries have been the most effective methods to treat chlorinated compounds in more transmissive aquifers involving lower initial concentrations (e.g., 100s to low 1.000s parts per billion) and presence of low levels of fine-grained soil.
- ISCR technologies such as ISBGT and ISRM require the presence of naturally occurring iron minerals such as pyrite and magnetite in the soil matrix, available dissolved sulfate, dissolved organic matter, and the presence of iron- and sulfate-reducing microbes to maintain iron/sulfate-reducing conditions.
- Treatability testing, including batch jar, column tests, or pilot tests, should be performed to determine types of amendments, dosages, and treatment efficacy. Results may be used to calculate reaction rates and residence time required to estimate the mass of reductants needed.
- Fine amendment particles (e.g., nanoscale ZVI) have greater surface area and therefore exhibit greater reactivity than large particles (e.g., microscale ZVI); however, the smaller particles will be expended faster. Various implementability issues have also been identified with the use of nano-scale ZVI, so microscale ZVI slurries are more commonly used.
- Injection-based applications require micro- or nano-scale ZVI slurries or chemical reagent solutions.
- Loss of media reactivity may occur due to passivation from adverse geochemical conditions and shorten the useful lifetime of a PRB. In addition, resulting precipitates can reduce the permeability of the PRB and/or formation. Additional applications of amendments or PRB media regeneration may be required. Data from treatability studies and site geochemistry should be considered for design.
- Strong reducing agents, such as sodium dithionate, can be introduced to the aquifer to reactivate previously injected ZVI, but there are limited data that clearly demonstrate the sulfidation process (micro- or nano-scale) to achieve this objective.
- Application of polysulfides may require the addition of a buffering amendment since they will oxidize to form sulfuric acid. Additional information pertaining to pH control and neutralization can be found here.
- Fine particles, such as nano-scale ZVI can reduce hydraulic conductivity of the aquifer, which can impact groundwater flow and treatment effectiveness. Larger particles, comprised of inert materials (e.g., sand), may be combined with the reactive materials to improve conductivity.
- Performance may decrease over time due to loss of porosity as a result of mineral precipitates or biofouling.
- Some hybrid amendments include ZVI emulsified within activated carbon, where contaminants can dissolve into the oil phase and come into direct contact for abiotic degradation by ZVI. The carbon source can also contribute to subsurface reducing conditions conducive to synergistic biotic degradation. Additional information on the in situ application of activated carbon amendments can be found here.
- Abiotic degradation may be difficult to monitor and distinguish from biotic degradation due to the lack of accumulation of some common intermediates (for example cDCE or vinyl chloride) and the short persistence of others, such as acetylene.
- Consideration should be given to the potential for reduced metals to be reoxidized and remobilized if groundwater becomes oxic, either downgradient and/or if reducing agents fail to maintain anoxic conditions within the treatment area prior to achieving remedial goals.
- Application of ISCR can reduce arsenic from the less mobile As5+ to the more mobile (and toxic) As3+ form. Oftentimes, mobilization is temporary, and will return to background levels outside of the treatment zone. In addition, if ZVI is used, arsenic will bind with ZVI corrosion products to form insoluble oxyhydroxides and arsenopyrites. If other types of reductants are used (e.g., calcium polysulfide), it may be necessary to add iron (e.g., ferrous sulfate) in order to sequester the arsenic, and bench-scale treatability tests should be performed.
Resources
Chang MC, Shu HY, Hsieh WP, Wang MC. Using Nanoscale Zero-Valent Iron for the remediation of Polycyclic Aromatic Hydrocarbons Contaminated Soil. Journal of the Air and Waste Management Association (2005)
This study evaluates the use of nanoscale ZVI to treat this study using nanoscale ZVI particles to remove pyrene contamination from soil.
EPA. Technology Focus on Permeable Reactive Barriers, Permeable Treatment Zones, and Application of Zero-Valent Iron
This EPA website provides an overview of PRB technologies, with links to additional resources.
EPA. In Situ Chemical Reduction
This EPA website provides an overview of ISCR technologies, with links to additional resources.
EPA. In Situ Chemical Reduction, Risk Management Research
This EPA website provides an introduction and several studies on using ISCR for treating arsenic, heavy metals, and acidity.
EPA. A Citizens Guide to In Situ Chemical Reduction (2012) (PDF) (2 pp, 1.09 MB)
A fact sheet intended for public guidance on ISCR to clean up pollution at Superfund and other sites.
SERDP-ESTCP. Remediation of TNT and RDX in Groundwater Using Zero-Valent Iron Permeable Reactive Barriers and Zero-Valent Iron In Situ Treatment Wells (2008)
This website contains the project report and cost and performance report for two demonstration studies, one replacing traditional well sand pack with coarse granular iron to treat munitions constituents, and a second using a ZVI barrier wall.
SERDP-ESTCP. Emulsified Zero-Valent Nano-Scale Iron Treatment of Chlorinated Solvent DNAPL Source Areas (2010) (PDF) (763 pp, 18.4 MB)
This cost and performance report describes applications of using nano-scale ZVI to treat DNAPL source zones.
SERDP-ESTCP. Chlorinated Solvent Source Zone Remediation, SEDRP-ESTCP Monograph (2014)
Chapter 10 of this book, titled In Situ Chemical Reduction for Source Zone Remediation, provides a thorough review of the category of ISCR technologies for both source zone and plume treatment.
Tratnyek, P.G., R.L. Johnson, G.V. Lowry, and R.A. Brown. In Situ Chemical Reduction for Source Remediation. Chapter 10 (pages 307-351) in Kueper B.K., HF Stroo, C Vogel, and CH Ward (Editors). Chlorinated Solvent Source Zone Remediation (2014)
This book provides an overview of ISCR technologies and provides guidance for applying the technology to treat chlorinated solvent source zones.
Xie, Yang and Cwiertny, David M. Use of Dithionite to Extend the Reactive Lifetime of Nanoscale Zero-Valent Iron Treatment Systems. Journal of Environmental Science and Technology. (2010)
This journal article describes a study to reactivate nanoscale ZVI with dithionite.
